19 Mar CRX100 Elicits Activity, Is Well Tolerated in Recurrent, Platinum-Resistant Ovarian Cancer
March 19, 2024
Russ Conroy, OncLive
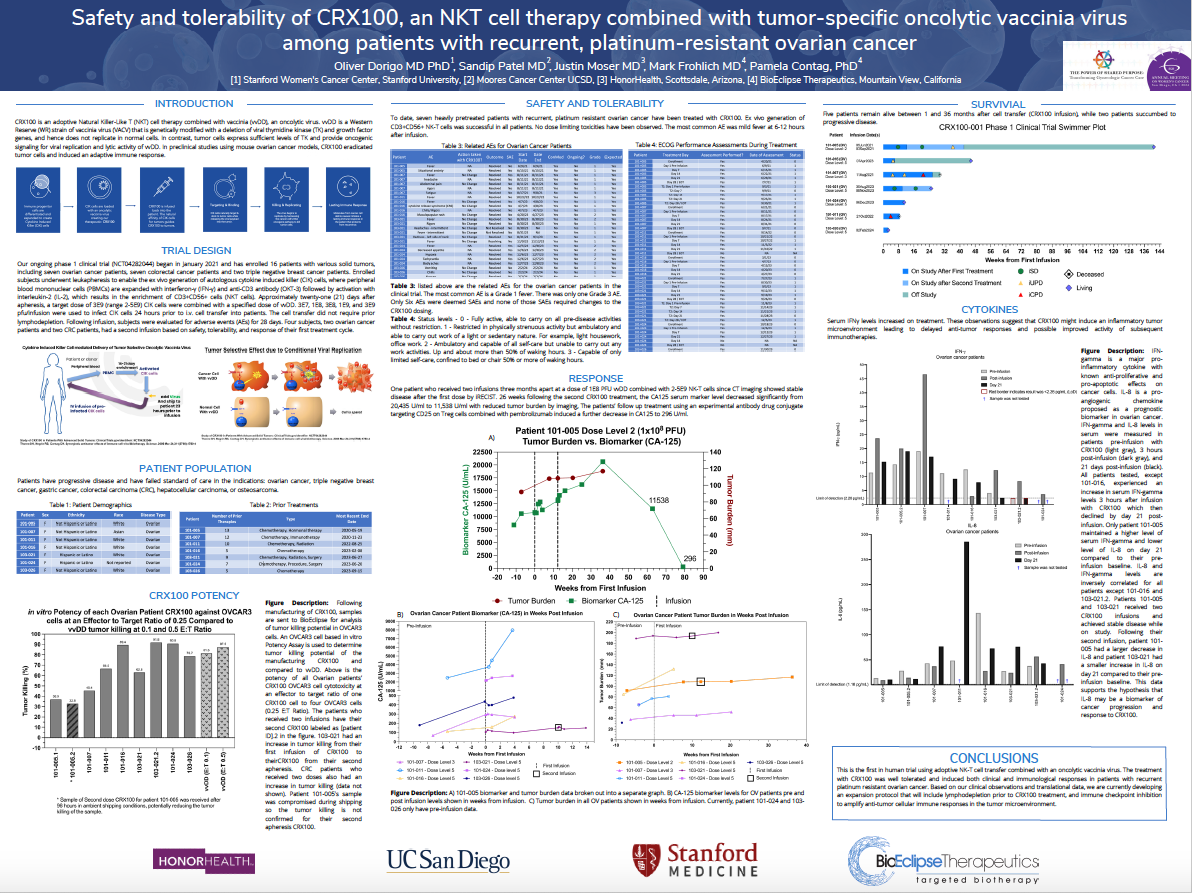
Combination treatment with the novel adoptive Natural Killer-Like T (NK-T) cell therapy CRX100 and a tumor-specific oncolytic vaccinia virus generated responses and was well tolerated in a small cohort of patients with recurrent, platinum-resistant ovarian cancer, according to data from a phase 1 trial (NCT04282044) presented at the 2024 SGO Annual Meeting on Women’s Cancer.1
Investigators reported stable disease in 1 patient who received an initial dose of 1E8 plaque-forming units (PFUs) of vaccinia plus 2-5E9 NK-T cells. Additionally, the patient had reduced tumor burden following a second dose of CRX100. This patient received subsequent treatment with an experimental antibody-drug conjugate plus pembrolizumab (Keytruda), which reduced CA125 serum marker levels to 296 U/mL.
Of 7 patients, 5 were alive at 1 to 36 months following treatment with CRX100. Two patients died due to progressive disease.
Findings highlighted that interferon-γ levels increased with study treatment, suggesting that CRX100 may produce an inflammatory tumor microenvironment that can delay antitumor responses and augment subsequent immunotherapy activity.
“This is the first-in-human trial using adoptive NK-T cell transfer combined with an oncolytic vaccinia virus,” Oliver Dorigo, MD, PhD, a Mary Lake Professor in Obstetrics & Gynecology – Gynecologic Oncology at Stanford Women’s Cancer Center, Stanford University and coauthors, wrote in the poster.